
Maximize family protection with most advanced technology
Exclusive Double Patented Technology
Provides Additional Protection Coverage
Can isolate and expand not just MSCs but also EpSCs from your baby’s umbilical cord tissue
CellOptima™ Patented Technology
Unlike conventional technology that can harvest Mesenchymal stem cells (MSCs) only, our exclusive patented breakthrough technology can isolate and expand not just Mesenchymal Stem Cells (MSCs) but also Epithelial Stem Cells (EpSCs) from your baby’s umbilical cord tissue.
About CellOptima™
More than 20 years ago, bone marrow was found to contain MSC then Wharton’s Jelly from the umbilical cord was found to contain MSC as well. More recently, a team of award-winning doctor and scientist from Singapore made significant inroad in the world with the discovery of two powerful stem cell types, MSC and EC, from cord lining.
Together, the Cambridge and Stanford University trained duo developed a unique technology known as CellOptima™, designed to harvest and multiply stem cells from cord lining.
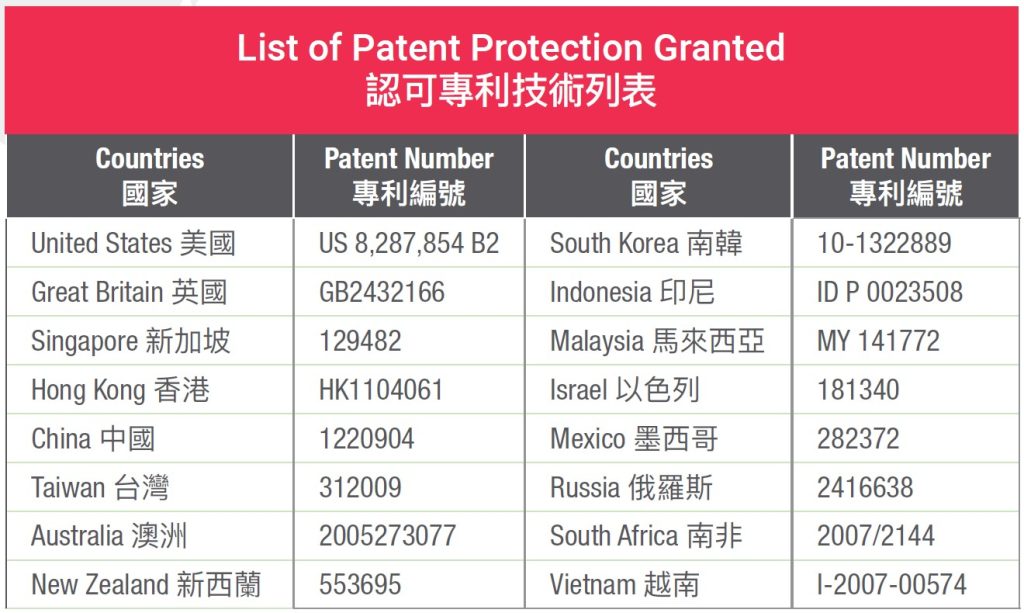
This revolutionary discovery subsequently led the team to receive more than 40 patent grants from 40 countries including United States, Europe, China, Singapore and Hong Kong. These patents prohibit anybody other than the patent owner from harvesting stem cells from cord lining.
CellOptima™ Assurances for you and your Family
Optimal Condition Assurance
Using CellOptima™ proprietary cell biomarker verifi cation, your baby’s cord lining will be tested after 4 weeks of cryopreservation. During this process, sample segments of the frozen cord lining will be thawed and MSC and/or EC will be harvested according to your choice of storage plan. This verifi cation step is important as it helps ensure that your baby’s cord lining has been properly stored and can be used for future medical treatments. The objective of this process is to validate the following:
- Cell type/s confi rmation using MSC and/or EC biomarkers
- Cell viability
- Cell proliferation also known as the ability to multiply further
- Cord lining sterility
Explant Purity & Dual Source Storage Assurance
If you choose to extract stem cells now from your baby’s cord lining whether MSC, EC or both for long-term storage, you will automatically enjoy complimentary storage of the cord lining in its original unexpanded form. This gives your family double access to both expanded stem cells which are “treatment-ready” as well as unmanipulated cord lining that can be used for multiple treatments and possibly the extraction of other cell types not identifi ed today. In addition to CellOptima™ proprietary cell biomarker verifi cation, the tests below will be done on the expanded stem cells to ensure that they are viable and safe for use.
- Count of cells
- Cell sterility testing
- Endotoxin analysis
- Mycoplasma analysis
Usability Assurance
Both MSC and EC harvested with CellOptima™ have been used successfully in human clinical applications. This gives you peace of mind knowing that what you are storing today, can really be used in the future to help your family.
Technology Accessibility Assurance
As long as you remain a cord lining client of HealthBaby, you are a guaranteed member of Global Cord Registry. As a member, you will have automatic access to CellOptima™ for the isolation and expansion of MSC and/or EC to support medical treatments. This membership is only granted to HealthBaby clients in Hong Kong.
Most Asked Questions by Parents Related to Cord Blood Banking
Until today, up to 113 diseases had been treated with cord blood transplants5. They could be divided into 7 categories, namely: Leukemia, Lymphomas, other Disorders of Blood Cell Proliferation, Inherited Disorders of Immune System, Inherited Metabolic Disorders, other Cancers and others.
Cord blood and umbilical blood contain two different stem cells which are widely adopted in different medical aspects. Cord blood is a rich source of HSCs which can create the blood and immune cells. Cancers, blood-related, immunity and metabolic diseases can be treated by cord blood. Umbilical cord is a rich source of MSCs which can create structural and connective tissues. Apart from minimizing GvHD in directed and unrelated allogeneic cord blood transplants, MSCs can potentially apply to chronic illnesses treatment, regenerative medicines as well as organ and tissue engineering5,6. Since cord blood and umbilical cord are rich sources of different types of stem cells, they may help to repair the body in different ways.
Entrusted by most parents in Hong Kong1, HealthBaby has a very comprehensive service coverage. Apart from the traditional liquid nitrogen storage plan, HealthBaby also pioneered to provide the advanced BioArchive® System. All storage plan of HealthBaby adopts the expensive liquid nitrogen as storage medium to provide the most stable tempertature for stem cell viability, providing the most quality umbilical cord and cord blood storage service.
To cater for different needs of clients, HealthBaby's cord blood and umbilical cord storage plan have very flexible and comprehensive service plan. Regarding cord blood plan, clients could select the storage plan base on the storage and processing system needs.
The umbilical cord and cord blood service fee already includes registration fee, processing fee and 18 years storage fee, no any additional charge during the contracted year. Extra discounted would be provided for registering both umbilical cord and cord blood storage plan. HealthBaby existing customers and other cord blood bank customers could also enjoy special offers. Please contact us for deatils and any enquiries.
For more details of cord blood service plan please refer to Service Plan.
More Questions?
Contact us through any method below:
Hotline: (+852)3188-8899 / (+853)2878-6717
WhatsApp: (+852)9660-8271 or Click Here
WeChat Official Account: HealthBaby 生寶臍帶血庫
Online: Click here to submit the enquiry online
Email: enquiry@healthbaby.hk





